electronic configuration of bromine|Iba pa : Baguio Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital. The sub-energy levels depend on the azimuthal quantum . Tingnan ang higit pa This Is Harness Racing Care and Training of the Trotter and Pacer Good for Business . U.S. ENTRIES AND RESULTS: updated Tue 9/3 16:11 PM: This web site is intended for personal, non-commercial use only. . Northfield Park: 2 F T, BUCKEYE STALLION SERIES 4th Leg: $80,000:
electronic configuration of bromine,Learn how to write the complete electron configuration of bromine (Br) using the Bohr model and the Aufbau principle. See the electron arrangement in different orbitals and subshells with examples and diagrams. Tingnan ang higit pa
Iba paThe total number of electrons in bromine is thirty-five. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in bromine in specific rules in different orbits and orbitals is called the electron configurationof . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital. The sub-energy levels depend on the azimuthal quantum . Tingnan ang higit pa
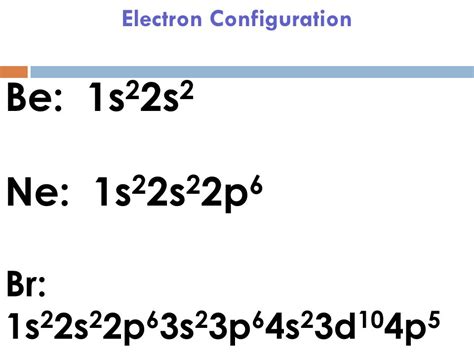
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons . Tingnan ang higit paAtoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of bromine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. In the bromine ground-state electron configuration, . Tingnan ang higit pa Bromine Electron Configuration. The properties of chlorine are intermediate between those of iodine and chlorine. Bromine Orbital Diagram. In this article today we are going to tell you about the .Bromine is the third halogen, being a nonmetal in group 17 of the periodic table. Its properties are thus similar to those of fluorine, chlorine, and iodine, and tend to be intermediate between those of the two neighbouring halogens, chlorine, and iodine. Bromine has the electron configuration [Ar]4s 3d 4p , with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens, it is thus one electron short of a full octet, and is hence a strong oxidising age. Learn how to write the electron configuration of bromine using the aufbau principle, periodic table, Bohr model, or orbital diagram. See examples, videos, and explanations for each method.

Electronic configuration of the Bromine atom. Valence electrons. Orbital diagram.Bromine is extracted by electrolysis from natural bromine-rich brine deposits in the USA, Israel and China. It was the first element to be extracted from seawater, but this is now .Bromine. Full electron configuration of bromine: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. selenium ← bromine → krypton.Physical and chemical properties of Bromine: general data, thermal properties, ionization energies, isotopes, reduction potentials, abundance of elements, crystallographic data. Schematic electronic configuration of bromine. The Kossel shell structure of bromine. Atomic spectrum. A representation of the atomic spectrum of bromine. Ionisation Energies and electron . The ground state electronic configuration of Br will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5.. excited state of Bromine electron configuration. The electronic configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6. In the excited state, Br will additionally require one electron to attain stability hence it will gain one electron to .Bromine has the electron configuration [Ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens, it is thus one electron short of a full octet, .electronic configuration of bromine Iba paIn this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic .
Bromine(Br): Bromine is a p-block element having an atomic number 35. Bromine belongs to group 17 which is known as halogens. Electronic configuration of Group 17: The elements of group 17 have seven electrons in their outermost shell. So, the valence electronic configuration of group 17 is n s 2 n p 5. Electronic configuration of Bromine: Explanation: All you need to do is work your way across the periodic table filling the orbitals as you go. The full version of this is: 1s22s22p63s23p64s23d104p5. Answer link. [Ar] 4s^ (2)3d^ (10)4p^5 All you need to do is work your way across the periodic table filling the orbitals as you go The full version of this is: 1s^ (2)2s^ (2)2p^ .
The electron configuration in the outer shell is \(ns^2np^5\). As the atomic number increases, the reactivity of the halogens decreases. Fluorine and chlorine exist as gases at room temperature, while bromine is a liquid, and iodine is a solid. Review. Pick two elements that are halogens. For each, write the name, chemical symbol, and atomic . What is the electron configuration of a bromide ion? Chemistry Electron Configuration Electron Configuration. 1 Answer anor277 Jul 20, 2017 Well, is it not isoelectronic with krypton..? Explanation: For atomic #Br#, we have #Z=35#, and thus for #Br^-# we gots 36 electrons to distribute in the usual way... Electron configurations are written using the principal quantum number n, followed by the orbital (s, p, d, or f) with the total number of electrons written as a superscript. Example: 1s 2 For writing ground state electron configurations, a few main steps should be followed. Find the amount of electrons in the atom. Example: Na: 11 e .The electronic configuration of Bromine will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. How do you write the electron configuration for Bromine? The electronic configuration of Bromine will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. Signup for our weekly newsletter to get the latest news, updates and stay in the loop to find out what parents are talking . Bromine -. Br: properties of free atoms. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The ground state electron configuration of ground state gaseous neutral bromine is [ .
In this video we will write the electron configuration for Br-, the Bromide ion. We’ll also look at why Bromine forms a 1- ion and how the electron configura.The Electron configuration of bromine is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵. Bromine, also known as liquid fire, is defined as a chemical element that is part of the periodic table of elements. Its atomic number is 35, it is located in the group of halogens, more precisely in group VII A. Br is its atomic symbol.
Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure.The chemical symbol for Bromine is Br. Electron Configuration and Oxidation States of Bromine. Electron configuration of Bromine is [Ar] 3d10 4s2 4p5. Possible oxidation states are +1,3,5/-1. Electron .

Bromine, element symbol Br, has an atomic number of thirty-five. One can find bromine, a halogen, in the p-block, group 17, particularly in period 4. Bromine is between chlorine and iodine, and has reactivity between the two. Bromine’s electron configuration is [Ar] 3d10 4s2 4p5. Bromine has 7 valence electrons, rendering it a highly .electronic configuration of bromineBromine, element symbol Br, has an atomic number of thirty-five. One can find bromine, a halogen, in the p-block, group 17, particularly in period 4. Bromine is between chlorine and iodine, and has reactivity between the two. Bromine’s electron configuration is [Ar] 3d10 4s2 4p5. Bromine has 7 valence electrons, rendering it a highly .Symbols used in the table of constants; Symbol Meaning; State: electronic state and / or symmetry symbol: T e: minimum electronic energy (cm-1): ω e: vibrational constant – first term (cm-1): ω e x e: vibrational constant – second term (cm-1): ω e y e: vibrational constant – third term (cm-1): B e: rotational constant in equilibrium position (cm-1): α e: rotational . Bromine -. Br: properties of free atoms. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The ground state electron configuration of ground state gaseous neutral bromine is [ Ar ]. 3d10. 4s2. 4p5 and the term symbol is 2P3/2. Schematic electronic configuration of bromine.
electronic configuration of bromine|Iba pa
PH0 · writing electron configurations for ions
PH1 · valence electrons of bromine
PH2 · pb 4+ electron configuration
PH3 · full electron configuration of br
PH4 · electronic structure of bromine
PH5 · electron configuration lab answers
PH6 · electron configuration chart
PH7 · br electronic configuration
PH8 · Iba pa